26th September, 2012 - 7:53 am
Categories:
IP Litigation
IP Practice in India
News & Updates
Pharma/Biotech Patent Litigations
26th September, 2012 - 7:53 am
Categories:
IP Litigation
IP Practice in India
News & Updates
Pharma/Biotech Patent Litigations
 0 Comments
0 Comments
In continuation of our previous post here, and following the availability of the 275 page judgement, we would discuss herein the various facets of the case and discuss one by one. This case actually involved two main issues as follows,
Issue I. Whether Roche’s Indian Patent 196774 is invalid (liable to be revoked under S. 64);
Issue II. Whether Cipla’s manufacturing, marketing and sale of Erlocip infringes the Patent IN196774
Let’s first discuss the issue of the validity of the IN’774 patent (which runs up to 181 pages of the judgement!)
The defendant (Cipla) prayed for revocation of the IN‘774 patent by way of counter claim on the grounds mentioned in S. 64 of the Indian Patent Act, namely, obviousness (lack of inventive step), anticipation by prior publication and prior claim, insufficient disclosure, false representation, violation of section 8 and section 3(d).
Out of these, the obviousness ground was discussed and argued in detail by Defendant and Plaintiff.
GROUND OF OBVIOUSNESS (LACK OF INVENTIVE STEP)
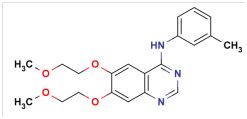
According to Cipla, arriving at the IN‘774 patent is obvious to the person skilled in the art. The structure of Erlotinib Hydrochloride as claimed in IN‘774 is:
IN‘774
And the structure of the compound identified as ‘Lead Compound’ by Cipla for their arguments on obviousness analysis is the one disclosed in EP’0566226 (‘226 patent) is:
EP‘226 patent: Example 51
Cipla contended that substitution of methyl group with ethynyl group at the third meta position would be obvious to a person skilled in the art.
The following points were main points of discussion:
- Selection of Lead Compound: Whether Example 51 is the Lead Compound or not?
- Motivation to substitute at 3 prime position
- Interchangeability of methyl and ethynly groups
1. Selection of Lead Compound: Whether Example 51 is the Lead Compound or not?
Cipla’s contentions:
- Cipla contended that there is sufficient motivation to do further R&D in the preferred compounds disclosed in EP‘226(patent which is an admitted prior art in the complete specification of suit patent) which discloses a class of quinazoline derivatives having anti-cancer properties.
- The compounds disclosed in EP‘226 are compounds which are obvious to try permutations and combinations on.
- EP‘226 discloses 3 preferred compounds amongst which one is example 51 which is the closest prior art.
Roche contentions:
Roche very rightly contended that Cipla has not explained as to how the said EP‘226 will act as a motivation towards arriving at the suit patent invention. Following are some of the main arguments by Roche:
- Cipla only relied upon the prior arts stated by Roche in their own patent ‘774 patent, all of which disclose -4-anilinoquinazoline derivative compounds possessing anti-cancer properties. Each of these prior arts, EP‘722, EP‘226, EP‘851, EP‘507, and EP‘498 have same core structure and are represented by Markush Structure thus encompassing millions of compounds.
- The compounds tested in prior arts EP‘722, EP‘851, EP‘507, and EP‘498 were found to have better or similar IC50 values as compared to compounds in EP‘226. The person skilled in the art will in particular look at Example 2(5), disclosed in the EP‘851 which has the IC50 value of 1nm.( Lower the IC50 value, more potent is the drug)
- Cipla has provided absolutely no evidence to show why EP’226 is the starting prior art as opposed to EP’851. Cipla has selected Example 51 with full knowledge of the structure of Erlotinib Hydrochloride, i.e. the defendant has selected Example 51 as the lead compound purely on the basis of hindsight.
- EP‘226 discloses 80 examples providing 102 specific exemplified compounds, 32 specifically preferred compounds, 18 claimed compounds and five prominent compounds for which specific IC50 values are given. Example 51 does not feature amongst the five prominent compounds mentioned in EP‘226 for which IC50 value have been provided. Therefore, there is no teaching, suggestion or motivation in EP‘226, regarding any useful properties or potent and promising activity to select Example 51 as the lead compound.
2. Substitution at 3 prime position
Cipla’s contentions: Cipla provides evidence to show that substitution at the third meta position of example 51 can be tried and made following the concept of Bio-isosterism.
Roche’s contentions: Roche contended thatout of all the available substitutions, methyl is kept constant in 3’ position in 80% of the prominent compounds for which specific IC50 values are given and thus the teachings of EP‘226 direct a person skilled in the art that 3’-Methyl should be left undisturbed for good biological activity.
3. Interchangeability of methyl and ethynly groups
Cipla’s contentions
Cipla contended that methyl and ethynyl are interchangeable due to: Bio-isosterism; and Interchangeability of methyl with ethynyl in light of prior arts US’590; EP’700; US’766; US’534.
Roche’s contentions
3’-position in Markush structure (R2) in EP’226 patent stands for 45 different substituents. There is no teaching provided that a person skilled in the art will substitute methyl with only Cyano group and not other 43 substituents as none of the 32 specific preferred compounds include the Cyano substitution.
As far as five prior arts were concerned, Roche contended that these could not be taken into record as they were not disclosed prior to the filing of the Replication to the Counter Claim.
Further Roche contended that of these five patent documents, US’766 and US’534 are not even valid prior arts under Section 64(1)(f) because they were published subsequent to the priority date of the ‘774 patent.
Judge’s Remarks on obviousness
Justice Manmohan Singh rightfully concluded that on the basis of evidence by defendant (Cipla) to establish obviousness, no ground of obviousness is made out under S. 64(1)(f).
GROUND OF VIOLATION OF SECTION S. 3(d)
The onus was on the defendant (Cipla) again to show as to how the plaintiff‘s (Roche’s) compound of Erlotinib Hydrochloride is a new form of known substance.
According to Cipla, Erlotinib is a derivative of Quinazoline, which is known for its anti-cancer activity with no enhanced activity and hence is hit by S. 3(d).
Plaintiff contended that “Quinazoline Derivatives” is a wide class of compounds having diverse uses including non-pharma uses and Erlotinib compound is just one specific compound of this family. Thus merely the term “Quinazoline Derivatives” does not infer that the Erlotinib compound is a derivative (new form) of the known substance.
Roche also provided evidence to show efficacy by way of clinical trials and thus according to Roche even if the said derivative Ertolinib is found to be one of the forms of the EP‘226 patent, still the same being efficacious cannot be presumed to be same substance under Section 3(d). However it could not be proven during cross examination of the expert witness that whether the clinical trials were conducted on the polymorphic form b (which is the marketed product Tarceva) or the Erlotinib compound (comprising both Polymorphic A and B).
The Judge concluded that since Defendant failed to give evidence that Erlotinib compound is a new form of known substance, the ‘774 is not hit by S. 3(d).
GROUND OF VIOLATION OF SECTION 8
A crisp summary of the discussion on this ground is provided herein. The few points are worth mentioning:
1. The IN’774 patent: filed on 13.3.1996; claims combination of polymorph A and B; granted in 2006.
2. The IN’507 application: filed on 1.6.2006; claims polymorphic B version of Erlotinib compound (and which is the marketed drug under name Tarceva); rejected by Controller in 2008 in pre-grant opposition proceedings.
3. US’221 is a corresponding granted patent to IN’507; filed in 2000.
Section 8 mandates the submission of information of corresponding Foreign applications (Form 3) on “same or substantially the same” inventions relating to the Indian application during the pendency of the Indian Application.
In this case, the plaintiff did not furnish Form 3 containing information of US‘221 patent. They stated that US‘221 patent is a distinct invention covering different compound. The Judge concluded that since US‘221 patent relates to “same or substantially the same” invention being the polymorphic form B of compound claimed in IN‘774 patent and hence the plaintiff was required to comply with section 8 by providing information of the same. This was the only ground of revocation which was in favour of Cipla.
OTHER GROUNDS
Cipla presented other grounds in its counter claim too, namely
- Ground of concealments and false representation under Section 64 (1)(j); and
- Lack of Title/Ownership in respect of plaintiffs
After the detailed discussion on the grounds, very briefly the Judge’s findings are summed up as below:
“The defendant failed to prove that that there were concealments made in relation to the prosecution before the patent office or there is an improper examination done by the patent office. Therefore, the examination and investigation process cannot be called into question by the defendant……..The challenge on the ground of ownership title and other concealments have not been established by the defendant by not discharging the onus casted upon it. No issue was framed. No evidence was led by the defendant.”
FINAL CONCLUSION BY THE JUDGE ON VALIDITY OF THE PATENT
According to Judge,
“There lies a discretion in the Court to proceed to revoke or not to revoke the Patent by way of usage of term the word “may” under Section 64 of the Act.”
In the present case, only one ground namely violation of section 3 is made out. However, the inconsistency on the part of defendant and the fact that no other ground relating to the revocation of the patent is satisfied under Section 64, made the Judge to exercise discretion in favour of the plaintiff. The inconsistency of the defendant lies in that the defendant at one instance, to resist infringement, argued that the compounds in US ‘221 and IN’774 are distinct and at other instance to prove violation of Section 8, argued that they are substantially the same.
The Judge thus finally concluded the IN’774 patent to be valid.
In the second part which follows soon, we would discuss the actual issue of the infringement of the IN’774 by Cipla in detail where the Judge observed that it was scientifically proven that Cipla’s Generic Drug is the Polymorphic Form B which is not Roche’s patented Drug and thus Cipla did not infringe said patent in India.
About the Author: Meenakshi Khurana, Patent Attorney, available at meenakshi@khuranaandkhurana.com